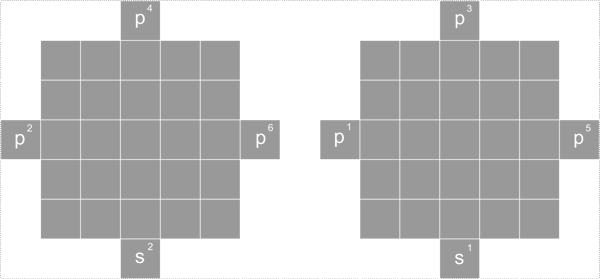
Pattern 10 distribution (10 proton rule)
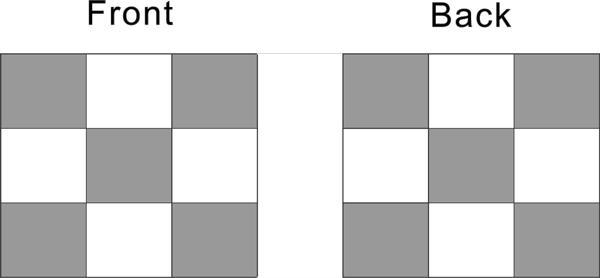
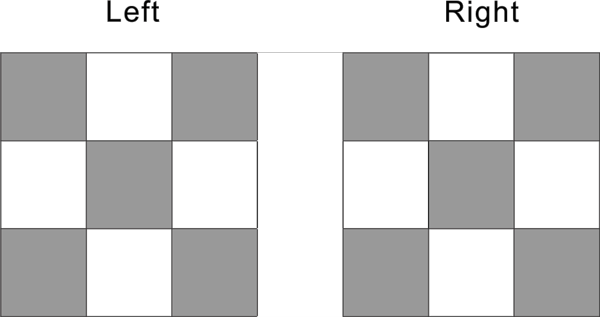
Beside the top and bottom, the cubic nuclide has 4 side face, front and back, left and right.
Each side face has full 5 proton distribution as shown in the diagram below

Front and back 2 side can have a total of 10 proton, left and right two side total can have 10 proton, pattern 10 proton is two side proton, each side has 5 protons.
Pattern 10 proton distributions is
d1 d2 d3 d4 d5 d6 d7 d8 d9 d10
Pattern 4 distribution (4 proton rule)
4 proton distributions is
f1 f2 f3 f4
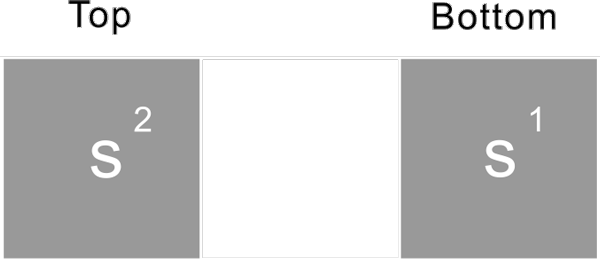
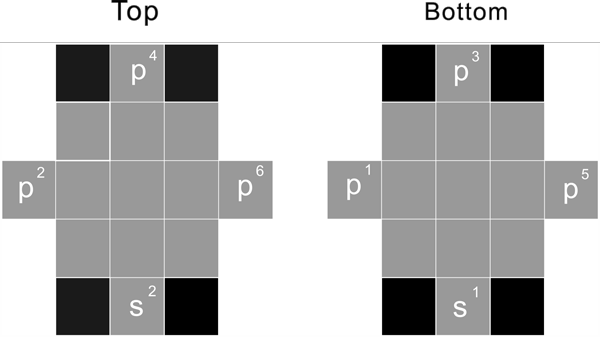
First period
s1 s2
The first period, has only two proton, s1 and s2. To fill the first period element, first the proton s1, becomes hydrogen, the second s2 then forms the helium nuclide.
-s2 as following
Second period
s1 s2 p1 p2 p3 p4 p5 p6 p3 p4 p5 p6
For the 2nd period, start to fill the 2 S proton, s2 as of Be-2, then start to fill all 6 P proton, s2p6, as of Ne-10
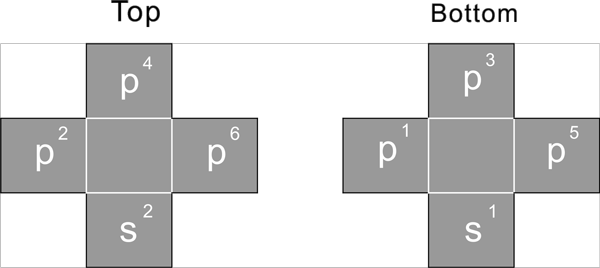
These 8 proton constitute the cube's 8 corner position
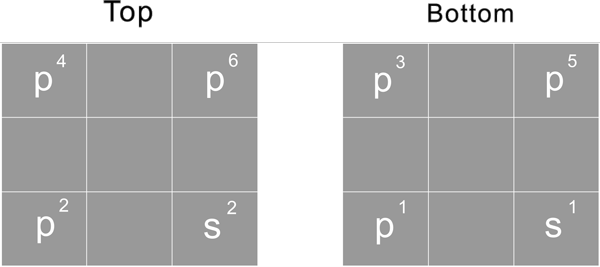
Third period
s1 s2 p1 p2 p3 p4 p5 p6 p3 p4 p5 p6
For the 3rd period, start to fill 2 S proton, s2 as of Mg-12, then start to fill all 6 P proton, s2p6 as of Ar-18
These 8 proton constitute the nuclide 8 corner unit
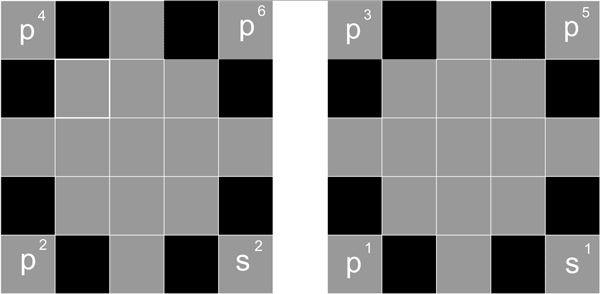
Fourth period
s1 s2
d1 d2 d3 d4 d5 d6 d7 d8 d9 d10
p1 p2 p3 p4 p5 p6 p3 p4 p5 p6
Starting from the fourth period, the proton begins to fill the nuclide 4 side D position.
For the fourth period, first fill the S2 protons, s2, as of Ca-20, and then fill the front side 5 D position, - s2 d5 as of Mn-25, then fill the back side 5 position, s2 d10, as of Zn-30, then start filling the 6 P proton, s2 d10 p6, as of Kr-36.
Pattern 10 Proton distribution
Due to S proton start to fill the D position. There are 3 exceptions
Cr-24 -- s1 d5;
Ni-28 -- s1 d9;
Cu-29 -- s1 d10;
Fifth Period
s1 s2
d1 d2 d3 d4 d5 d6 d7 d8 d9 d10
p1 p2 p3 p4 p5 p6 p3 p4 p5 p6
Very similar to the fourth period, start with the fifth period, first fill the two S protons, s2 as of Sr-38, then fill the left side 5 D position, s2d5, as of Tc-43, then fill the right side 5 position, s2d10 as of Cd-48, then fill the 6 P position, - s2d10 p6 as of Xe-54
Pattern D10 distribution
Due to S proton start to fill the D position. There are 5 exceptions
Nb-41 -- s1 d4;
Mo-42-- s1 d5;
Ru-44 -- s1 d7;
Pd-46-- d10;
Ag-47-- s1 d10;
Six period
s1 s2
f1 f2 f3 f4
d1 d2 d3 d4 d5 d6 d7 d8 d9 d10
d1 d2 d3 d4 d5 d6 d7 d8 d9 d10
p1 p2 p3 p4 p5 p6 p3 p4 p5 p6
From the sixth period, it starts to fill the 4f protons.
At the beginning of the six period, first fill the two S protons, the s2 as of Ba-56; and then fill all the four F protons, s2 f4 as of Nd-60; and then fill the front side 5 D proton s2 f4 d5, as of Tb-65; and then fill the back side 5 D proton , both front and back side total 10 protons, s2 f4 d10, as of the element Yb-70; and then to fill left side 5 D proton, s2 f4 d10 d5 as of Re-75; and then to fill right side 5 D proton, left and right total has 10 D proton, s2 f4 d10 d10, as of Hg-80; and to fill all 6 P proton, s2 f4 d10 d10p6 as of element Rn-86.
Pattern 10 distribution

There are two D10 distributions, one is for the cube's front and back side, a total of 10 protons; another D10 is for Cube's the left side and right side, total D10 proton.
Seventh Period
s1 s2
f1 f2 f3 f4
d1 d2 d3 d4 d5 d6 d7 d8 d9 d10
d1 d2 d3 d4 d5 d6 d7 d8 d9 d10
p1 p2 p3 p4 p5 p6 p3 p4 p5 p6
Very similar to the six period, for the seventh period, first fill the two S protons, s2 of Ra-88; and then fill all the four F protons, s2f4 as of U-92; and then fill the front side 5 D proton s2 d5 as of Bk-97; and then fill the back side 5 D proton, both front side and back side, a total of 10 protons, s2 f4 d10 as of element No-102; and then fill left side 5 D proton, s2 f4 d10 d5 as of Bh-107; and then fill right side 5 D proton, a total of 10 D proton, s2 f4 d10 d10 as of Uub-112, and then to fill all 6 P proton, s2 f4 d10 d10 p6 as of the element Uuo-118.
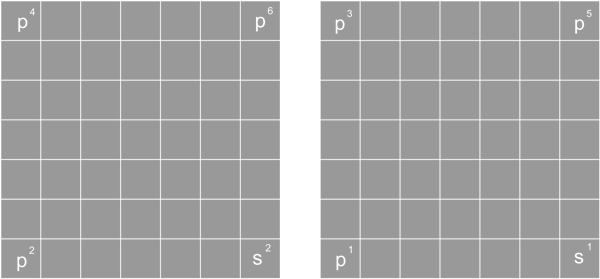
Pattern 10 D distribution

