The left and right side in total can fill another 10 proton.
Pattern 10 proton distribution
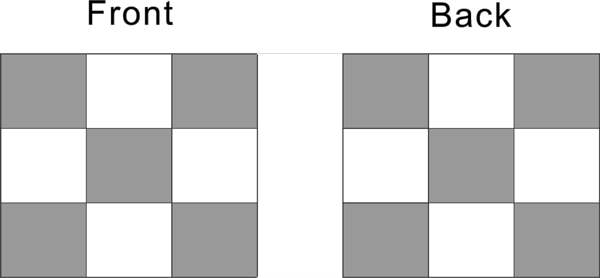
Front the side and back side, in total can fill 10 protons.
The left and right side in total can fill another 10 proton.
Pattern 10 proton distribution

On the periodic table of elements, Pattern 10 proton form the D block as shown below
Period |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
4* |
|
Sc |
Ti |
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
Zn |
5* |
|
Y |
Zr |
Nb |
Mo |
Tc |
Ru |
Rh |
Pd |
Ag |
Cd |
6* |
# |
Pm |
Sm |
Eu |
Gd |
Tb |
Dy |
Ho |
Er |
Tm |
Yb |
|
|
Lu |
Hf |
Ta |
W |
Re |
Os |
Ir |
Pt |
Au |
Hg |
7* |
& |
Np |
Pu |
Am |
Cm |
Bk |
Cf |
Es |
Fm |
Md |
No |
|
|
Lr |
Rf |
Db |
Sg |
Bh |
Hs |
Mt |
Ds |
Rg |
Uub |
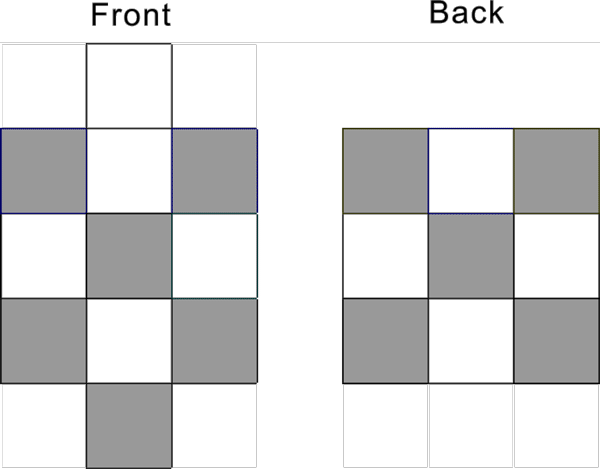
From period 4 to period 7, there are a total of 60 pattern 10 protons within D block.
Pattern 10 D protons are located on the four side face of the nuclide (excluding the top and bottom face).
At the beginning of the period 4, the protons begin to fill the D proton, a total of four sides, front and back sides, right and left sides.
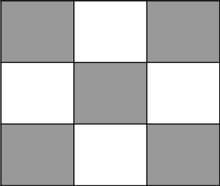
Each side can fill five protons, with a face centered proton structure as shown below:

For the pattern 10 proton, first fills the five protons of the front side, and then fills five D proton on the back side. The pattern 10 D protons distribution is shown below:
d1 d2 d3 d4 d5 d6 d7 d8 d9 d10
d0
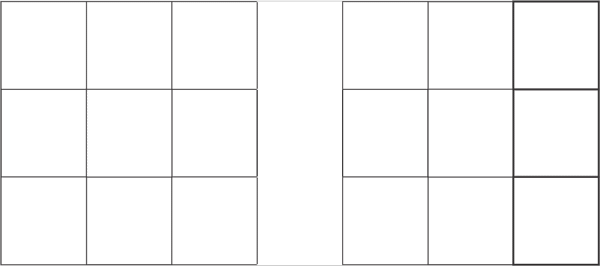
d1
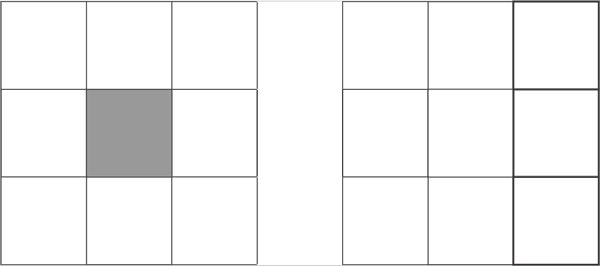
d2
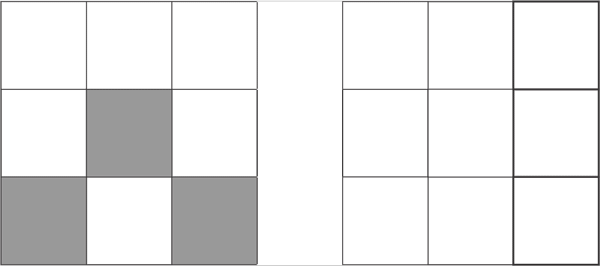
d3

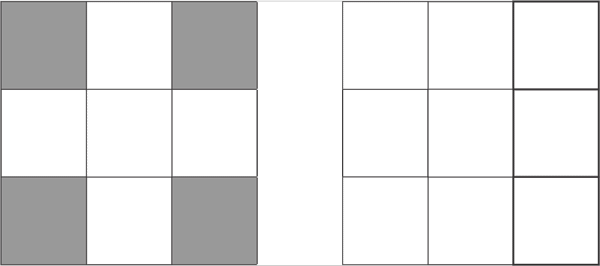
d5
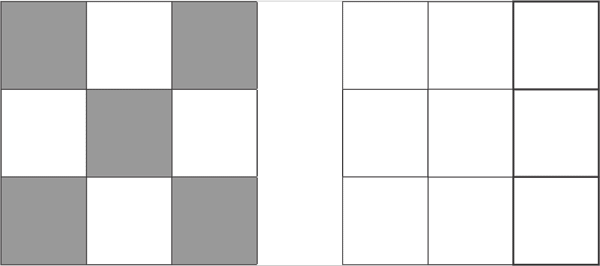
d6
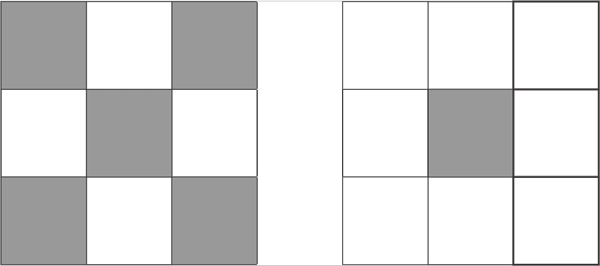
d7
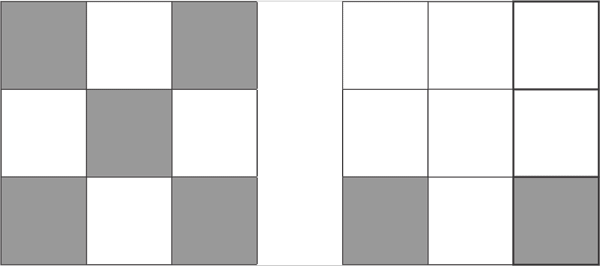
d8
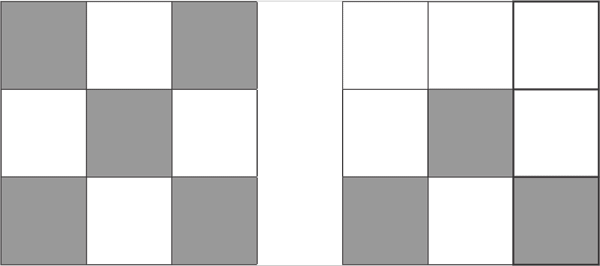
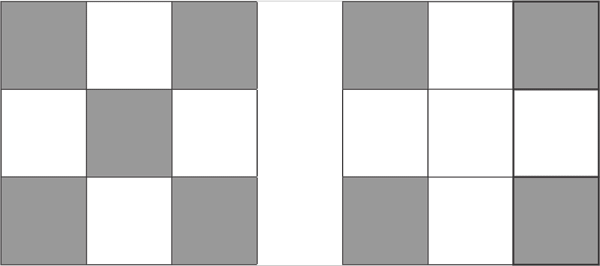
d10
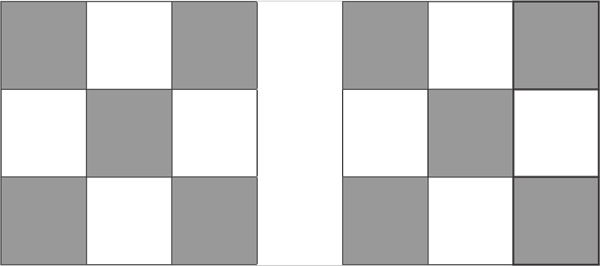
On the column 8, the last column on the D block, all fully fill the pattern 10 proton d10
For the pattern 10 D proton, due to the 2 S proton start to fill the D proton. There are exceptions for the D proton which do not exactly follow the sequence of d1 to d10.
The column 9 element Cu-29, Ag-47 and Au-79, should have the s2d9 configuration, but due to one S proton which left their S position to start to fill the D proton, their proton configuration become s1d10.
The column 8 element, Ni-28 and Pt-78, should have the s2d8configuration, but due to one S proton which left their S position to start to fill D position, their proton configuration become s1d9.
The column 8 element Pd-46, should have the s2d8 configuration, but due to 2 S proton which left their S position to start to fill the D position, their proton configuration become d10
The columns 5 elements, all fully fill the one side D 5 proton and they have the s2d5 configuration.
The column 4 element Cr-24, Mo-42, should have the s2d4 configuration but due to one S proton which starts to fill the D block, their configuration become s1d5.
The column 3 element Nb-41, should have the s2d3 configuration, due to one S proton which starts to fill D position, their configuration become s1d4
Here is the list of exception for the D block proton
Element |
Column |
Configuration |
Nb-41 |
D3 |
s1d4 |
Cr-24, Mo 42 |
D4 |
s1d5 |
Ru-44 |
D6 |
s1d7 |
Ni-28, Pt-78 |
D8 |
s1d9 |
Pd-46 |
D8 |
d10 |
Cu-29, Ag-47, Au-79 |
D9 |
s1d10 |
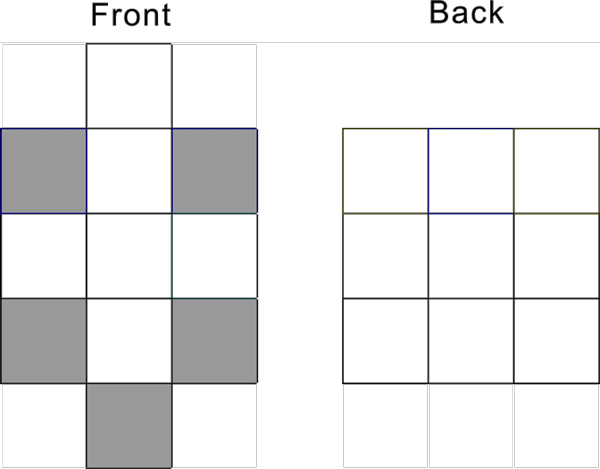
Nb-41 S and D proton configuration s1d4 has the following pattern :
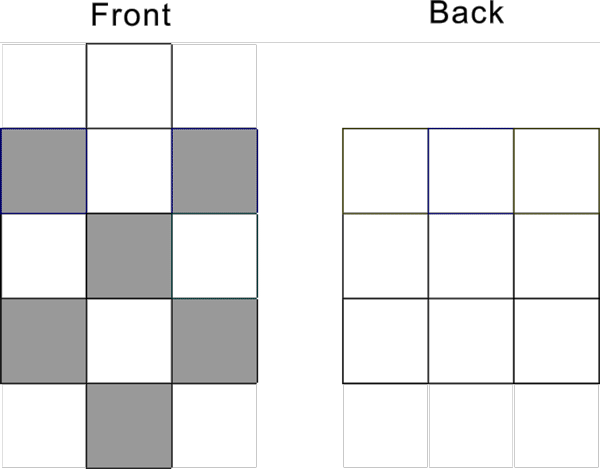
Ru-44 configuration s1d7 has the following pattern:
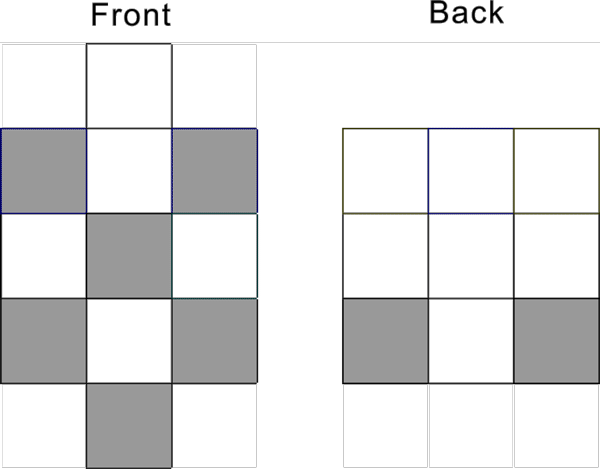
Ni-28, Pt-78 configuration s1d9 has the following pattern:
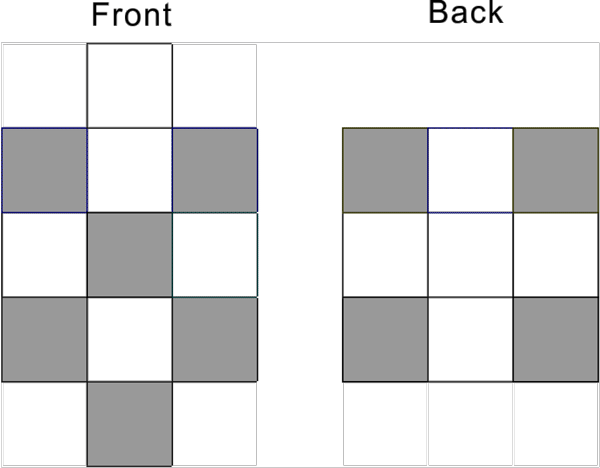
Pd-46 proton configuration d10 has the following pattern:
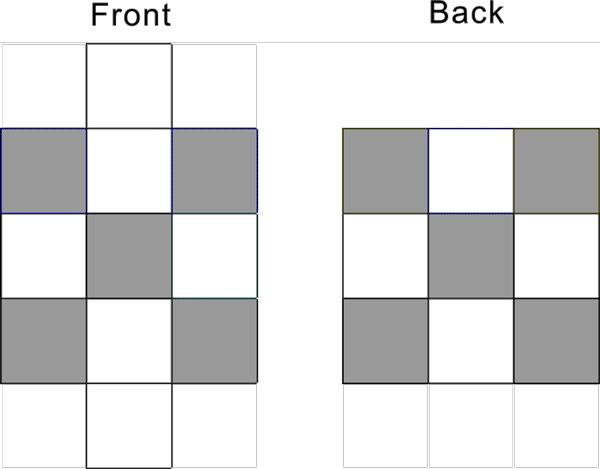
Cu-29, Ag-47, Au-79 proton configuration s1d10 has the following pattern: